| Data source |
Cardiovascular Research: cvab004
|
| Description |
This study reveals that the cardiac ECM exhibits regional specialization required for heart morphogenesis, and sheds light on how embryonic left–right asymmetry acts in concert with ECM regionalization to fine tune heart shape. This work can help us understand the origins of congenital heart defects, and in particular the nature of morphological heart abnormalities in patients with heterotaxia-associated heart malformations. Furthermore, recent studies suggest the ECM is a key regulator of regenerative potential in the heart, thus defining how distinct ECM composition impacts upon heart form and function has implications for developing regenerative therapies in the future. |
| Key word |
heart morphogenesis; extracellular matrix; laterality; zebrafish; heart development |
| Publication |
Derrick, C. J. , et al. "Asymmetric Hapln1a drives regionalised cardiac ECM expansion and promotes heart morphogenesis in zebrafish development." Cardiovascular research :cvab004. |
| Abstract |
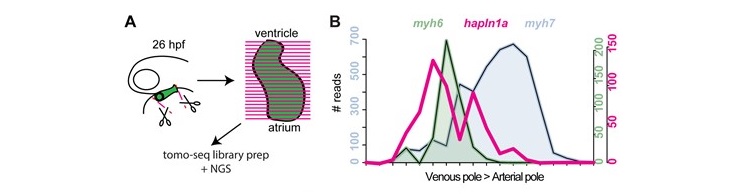
[Aims] Vertebrate heart development requires the complex morphogenesis of a linear tube to form the mature organ, a process essential for correct cardiac form and function, requiring coordination of embryonic laterality, cardiac growth, and regionalized cellular changes. While previous studies have demonstrated broad requirements for extracellular matrix (ECM) components in cardiac morphogenesis, we hypothesized that ECM regionalization may fine tune cardiac shape during heart development. [Methods and results] Using live in vivo light sheet imaging of zebrafish embryos, we describe a left-sided expansion of the ECM between the myocardium and endocardium prior to the onset of heart looping and chamber ballooning. Analysis using an ECM sensor revealed the cardiac ECM is further regionalized along the atrioventricular axis. Spatial transcriptomic analysis of gene expression in the heart tube identified candidate genes that may drive ECM expansion. This approach identified regionalized expression of hapln1a, encoding an ECM cross-linking protein. Validation of transcriptomic data by in situ hybridization confirmed regionalized hapln1a expression in the heart, with highest levels of expression in the future atrium and on the left side of the tube, overlapping with the observed ECM expansion. Analysis of CRISPR-Cas9-generated hapln1a mutants revealed a reduction in atrial size and reduced chamber ballooning. Loss-of-function analysis demonstrated that ECM expansion is dependent upon Hapln1a, together supporting a role for Hapln1a in regionalized ECM modulation and cardiac morphogenesis. Analysis of hapln1a expression in zebrafish mutants with randomized or absent embryonic left–right asymmetry revealed that laterality cues position hapln1a-expressing cells asymmetrically in the left side of the heart tube. [Conclusion] We identify a regionalized ECM expansion in the heart tube which promotes correct heart development, and propose a novel model whereby embryonic laterality cues orient the axis of ECM asymmetry in the heart, suggesting these two pathways interact to promote robust cardiac morphogenesis. |